Chinese scholars have made progress in the research on direct deaminative functionalization of aromatic amines
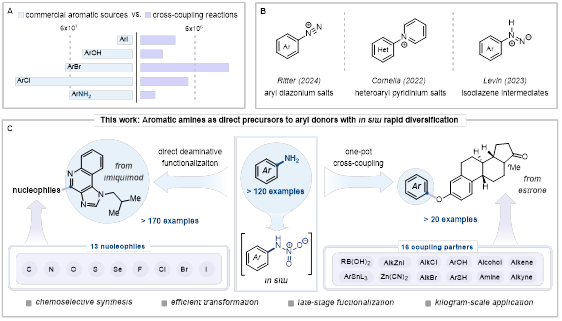
Figure. Deaminative functionalization of aromatic amines
Under the support of the National Natural Science Foundation of China (Grant Nos.: 22171049, 22122104), the team led by Xiaheng Zhang from the UCAS Hangzhou Institute for Advanced Study, in collaboration with the team led by Xiaosong Xue from the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, has achieved significant progress in the research on direct deaminative functionalization of aromatic amines. The related findings, entitled "Direct deaminative functionalization with N-nitroamines", were published online in the journal Nature on October 28, 2025. Paper link: https://www.nature.com/articles/s41586-025-09791-5.
Aromatic amines are vital building blocks widely present in pharmaceuticals, natural products and pesticides. Despite the abundance and versatility of aromatic amines as starting materials, general methods for their direct functionalization remain scarce. Conventional aromatic amine transformations rely heavily on the Sandmeyer Reaction, which requires the prior conversion of aromatic amines into unstable diazonium salts with explosion risks, and are also plagued by issues such as high copper consumption and poor substrate compatibility.
To address these challenges, the research team developed a strategy involving nitric acid-mediated formation of N-nitroamine intermediates, which directly activates the amino group, enabling the efficient transformation of aromatic C−N bonds into C−C bonds and various C−X bonds (X = Cl, Br, I, F, N, S, Se, O). This method employs only readily available simple reagents in the laboratory, demonstrating excellent versatility and applicability to diverse types of pharmaceutically relevant heteroaromatic amines and aniline derivatives with varied electronic properties and structural features. Furthermore, kilogram-scale preparation of products can be effortlessly achieved using this approach. To enhance operational simplicity and synthetic efficiency, the research team further developed a one-pot deaminative cross-coupling sequence, which integrates in situ generated aryl (pseudo)halides with transition-metal-catalyzed reactions, eliminating the need for intermediate isolation. This research provides a safer and economical potential alternative to the traditionally widely used yet explosive aryl diazonium chemistry, holding promising applications in numerous crucial fields such as pharmaceuticals and materials science.
Contact Us

National Natural Science Foundation of China
Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@nsfc.gov.cn
