Chinese Scientists Make Breakthrough in universal CAR-T therapy
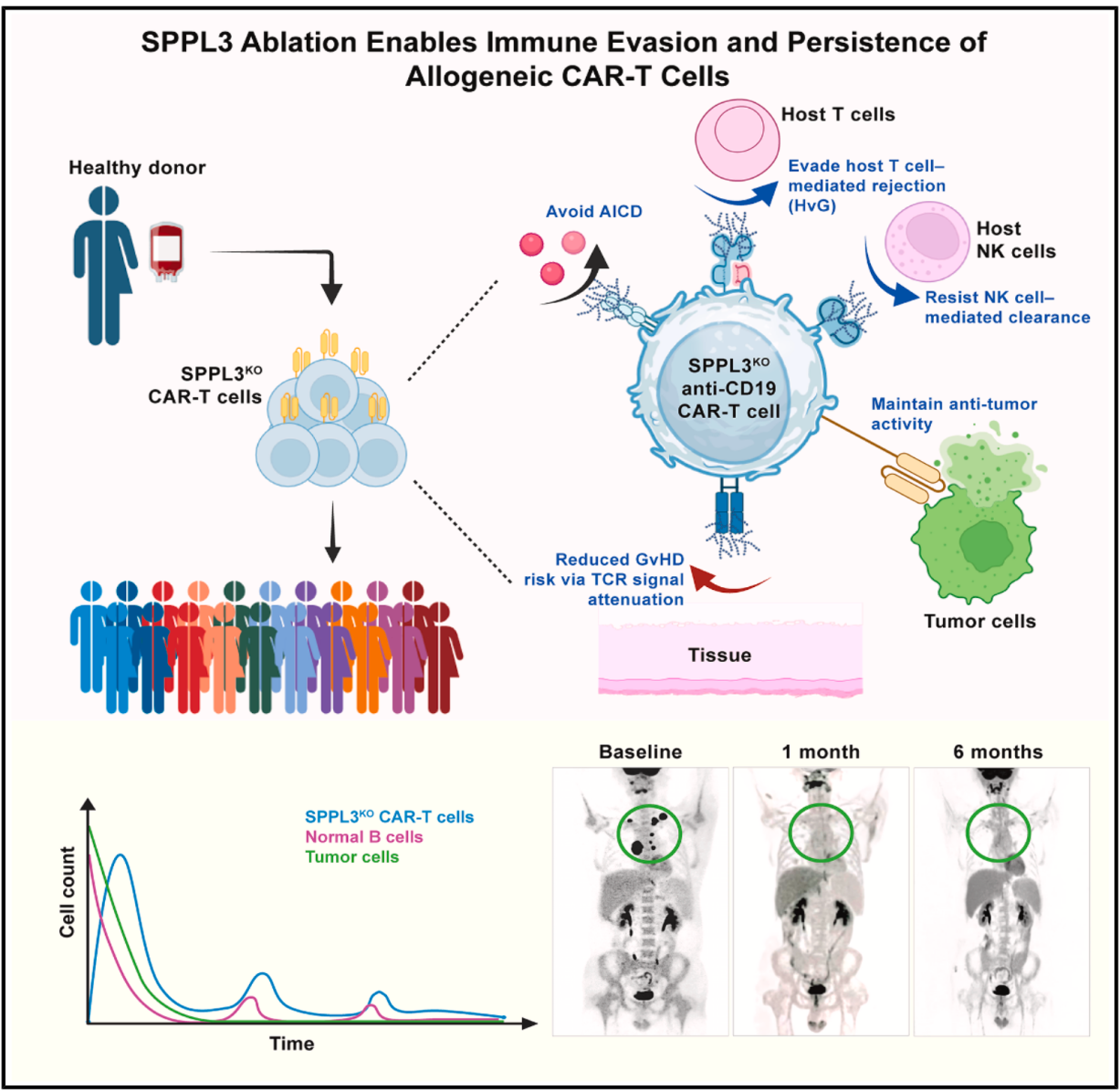
Figure: SPPL3 Ablation Enables lmmune Evasion and Persistence of Allogeneic CAR-T Cells
Supported by the National Natural Science Foundation of China (Grants No. 82341207, 82341208), a collaborative research, led by Professor Wensheng Wei from Peking University, Professor Weidong Han from the Chinese PLA General Hospital and EdiGene, has made significant advances in universal CAR-T therapy. This study, entitled “Glycan shielding enables TCR-sufficient allogeneic CAR-T therapy”, was published online in Cell on August 21, 2025 (Article link: https://www.cell.com/cell/fulltext/S0092-8674(25)00910-9).
Although autologous CAR-T cell therapy has transformed the treatment landscape for B-cell malignancies, this groundbreaking therapy faces challenges in logistics, product quality and patient eligibility. Universal CAR-T cells (also called allogeneic CAR-T cells), which generated from healthy-donor T cells and supplied as off-the-shelf drugs, promise scalable, on-demand manufacturing, but persistence and rejection in an allogeneic host remain the central bottlenecks.
Utilizing genome-wide CRISPR screening, the researchers identified signal peptide peptidase-like 3 (SPPL3). SPPL3 deletion increased the level and forms of glycosylation on primary T cells, forming a dense glycan layer (“glycan shielding”) that restricted ligand accessibility and reduced allogeneic immunity. Editing SPPL3 improved immune tolerance and persistence without compromising the functionality of anti-CD19 CAR molecules. In a phase I clinical trial, SPPL3-null, T cell receptor (TCR)-deffcient anti-CD19 allogeneic CAR-T cells reached the safety primary endpoint, without graft-versus-host disease (GvHD) observed in 9 patients with relapsed/refractory B cell non-Hodgkin lymphoma (B-NHL), but CAR-T persistence was limited. Reverse translational research highlighted the pivotal role of TCR in sustaining T cell persistence. We therefore evaluated the safety of SPPL3-null, TCR-sufffcient CAR-T therapy on three patients with lymphoma or leukemia for compassionate care and observed no clinical signs of graft-versus-host disease, and CAR+ T cells persisted for up to 6 months. Our findings suggest glycan shielding by SPPL3 deletion break through the allogeneic CAR-T immune barrier, while TCR signaling is indispensable for sustained CAR-T cell persistence and prolonged tumor control (Figure).
This research uncovered that SPPL3 editing not only preserved CAR-mediated anti-tumor efficacy but also significantly enhanced immune tolerance and persistence in vivo. It provides a clinically feasible blueprint for developing TCR-retaining universal CAR-T therapy, offering a novel paradigm for the design and development of universal CAR-T cell therapies.
Contact Us

National Natural Science Foundation of China
Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@nsfc.gov.cn
