Chinese researchers make progress in the field of noble metal based catalysts
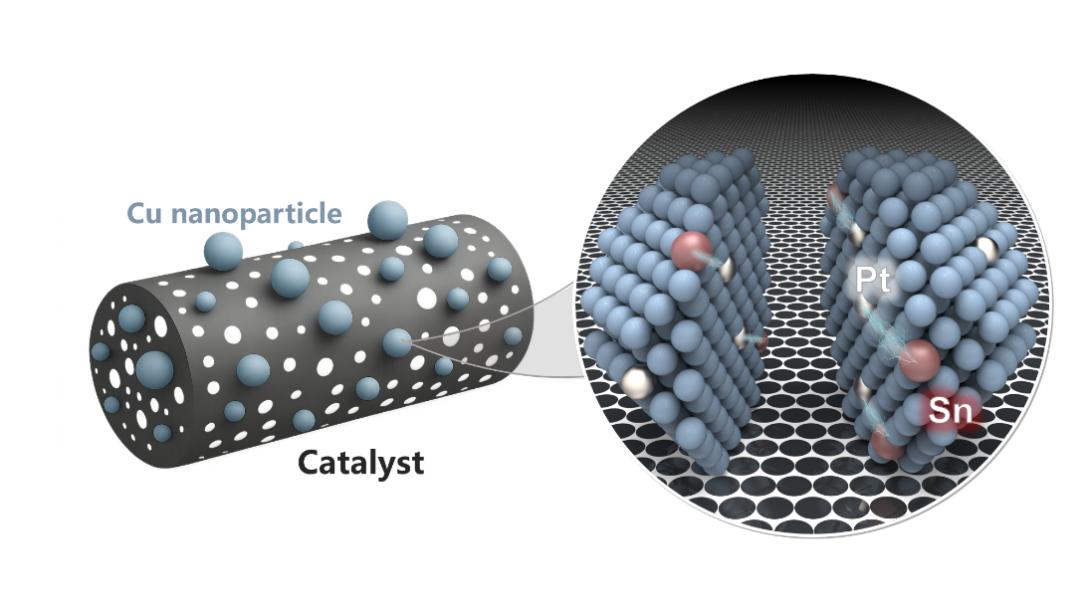
Figure. Schematic illustration of the “atom abstraction” process driven synergistically by differences in intermetallic atomic size and bond strength.
Supported by the National Natural Science Foundation of China (Grant Nos. 22121004, 22122808, U1862207, and 22408269), Professor Jinlong Gong’s team at Tianjin University has achieved a significant breakthrough in noble metal catalysis. Their latest research, titled “Full utilization of noble metals by atom abstraction for propane dehydrogenation,” was published online in Science on September 25, 2025. Article link: https://www.science.org/doi/10.1126/science.adw3053.
Noble metal catalysts are indispensable in the chemical industry, yet maximizing the atomic efficiency remains a long-standing challenge. Traditional approaches to disperse noble metals typically rely on strong metal–support interactions, which often result in partially oxidized metal atoms. While porous materials such as zeolites can stabilize metallic single atoms, their confined microporous structures hinder molecular diffusion, limiting their application in reactions involving large molecules. Achieving fully exposed, metallic single atoms on open surfaces has thus remained a formidable goal in catalysis.
To overcome this challenge, Professor Gong’s team developed an innovative “atom abstraction” strategy, leveraging the differences in atomic size and bond strength among metals. By introducing tin (Sn) atoms, with a larger atomic radius, into platinum-containing copper (Pt–Cu) nanoparticles, the Sn atoms preferentially segregate to the particle surface. The strong metallic bonding between Sn and Pt simultaneously drives Pt atoms toward the surface, thereby achieving a high degree of atomic dispersion across the nanoparticle. When applied to the industrially critical propane dehydrogenation (PDH) reaction, the resulting PtSnCu ternary alloy catalyst delivered a propane conversion rate comparable to that of the conventional industrial PtSnK/Al2O3 catalyst, despite using only one-tenth of the platinum content. Moreover, it demonstrated superior catalytic stability and propylene selectivity.
This study reveals the mechanism by which intermetallic synergy enhances the utilization efficiency of noble metal atoms. The proposed “atom abstraction” strategy offers valuable insights for the efficient use of scarce noble metal resources and the rational design of high-performance catalysts.
Contact Us

National Natural Science Foundation of China
Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@nsfc.gov.cn
