Chinese Scientists Achieved Photocatalytic Heterolytic Hydrogen Dissociation for CO2 Reduction to Ethylene
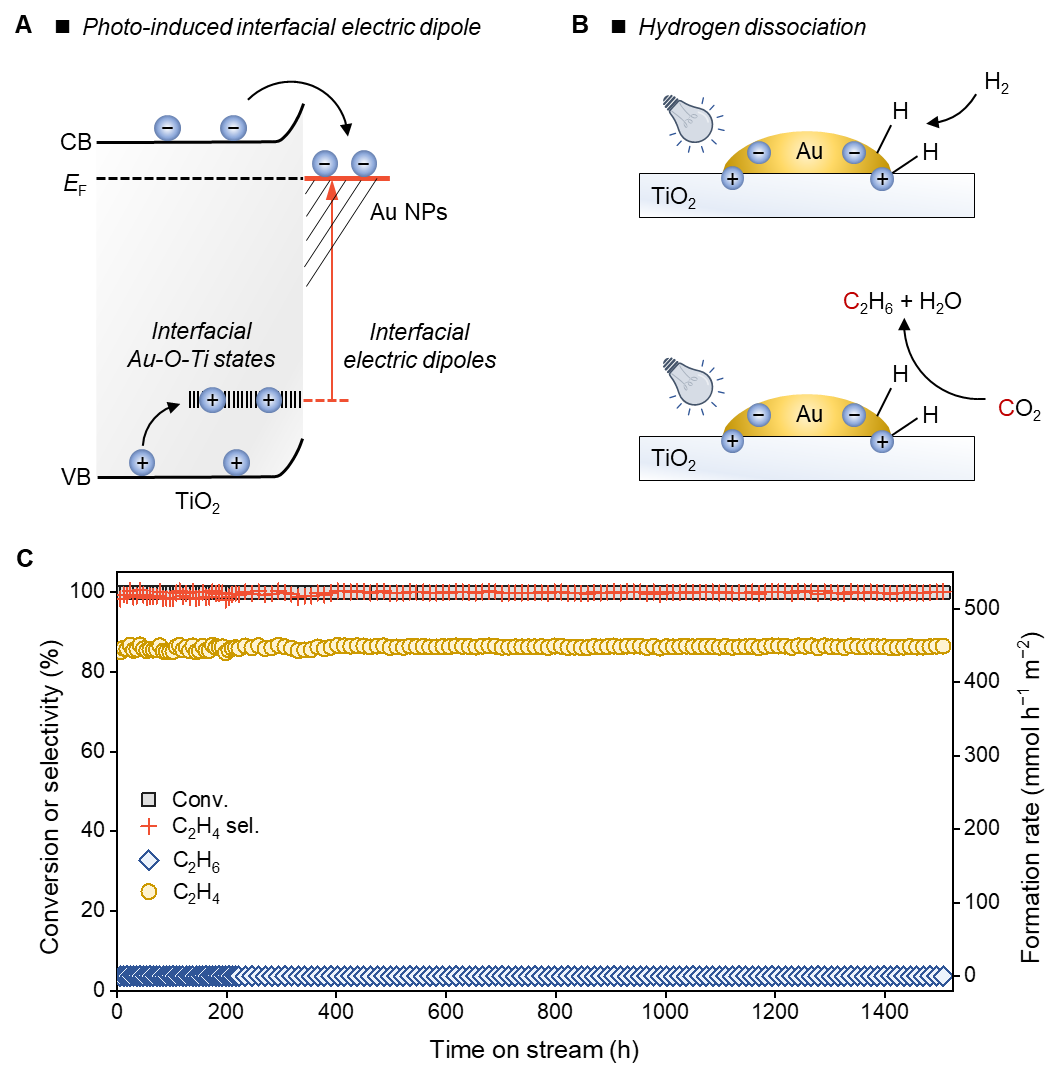
Figure: (A) Proposed mechanism for the generation of photogenerated electron-hole pairs in spatially proximal sites. (B) Schematic diagram of heterolytic hydrogen dissociation induced by the electron-hole pairs for CO2 hydrogenation. (C) Photochemical CO2 reduction to ethylene through light-induced CO2 hydrogenation to C2H6, followed by a second-step C2H6 dehydrogenation.
Supported by the National Natural Science Foundation of China (22025206 and 22172157), a research team composed of Prof. Wang Feng and Associate Prof. Luo Nengchao from Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and Prof. Paolo Fornasiero from the University of Trieste, Italy, has made progress in heterolytic hydrogen dissociation driven by photoenergy. Their research, titled "Photochemical H2 dissociation for nearly quantitative CO2 reduction to ethylene," was published in Science on September 4, 2025 (https://www.science.org/doi/10.1126/science.adq3445).
Hydrogenation is a crucial reaction in the chemical industry, with approximately 25% of reactions involving at least one hydrogenation step. This process involves homolytic dissociation of hydrogen into neutral hydrogen atoms and heterolytic dissociation into polar hydrogen species. Polar hydrogen species are highly reactive and selective toward the hydrogenation of polar functional groups, offering unique advantages in selective hydrogenation and activation of inert molecules. However, heterolytic hydrogen dissociation typically operates at high reaction temperatures and pressures. The fundamental reason is that the active sites of catalysts for heterolytic hydrogen dissociation are low-concentration surface defect structures with a low charge density.
To address this challenge, the team proposed a strategy that photogenerated electrons and holes were leveraged to form spatially proximal positive and negative charge centers, thereby constructing catalytic sites with improved concentration and charge density for heterolytic hydrogen dissociation. Using Au/TiO2 as a model catalyst, electrons generated by UV excitation of TiO2 migrate to Au nanoparticles and are trapped. Meanwhile, photogenerated holes are trapped by Au-O-Ti defect states at the interface between Au nanoparticles and the TiO2 support (Figure A). This creates improved concentration of electron-hole pairs trapped at spatially proximal sites, which effectively suppresses electron-hole recombination and improves heterolytic hydrogen dissociation efficiency. The team applied this photochemical hydrogen dissociation method to CO2 reduction, promoting the formation of C2 products, leveraging polar hydrogen species from hydrogen dissociation (Figure B). In a fixed-bed photoreactor, they achieved a single-pass CO2 conversion of nearly 100% and an ethane selectivity exceeding 99% over 1500 hours of irradiation. Furthermore, subsequent photocatalytic ethane dehydrogenation generated ethylene with a single-pass yield exceeding 99% (Figure C).
The proposed electron-hole pair mechanism for heterolytic hydrogen dissociation circumvents the trade-off between conversion and selectivity in hydrogenation reactions. This chemistry is expected to be used in hydrogenation reactions requiring ambient temperature and pressure, with the target of reducing energy consumption while improving hydrogenation safety, thereby promoting optimized utilization of carbon resources for carbon neutrality.
Contact Us

National Natural Science Foundation of China
Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@nsfc.gov.cn
