Chinese Researchers Make Progress in Synergistic Chem-Bio Synthesis of TCM Terpenoids
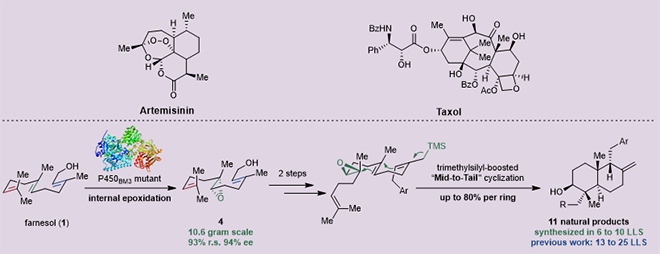
Figure Terpenoid drugs derived from Traditional Chinese Medicine and their concise and efficient total synthesis enabled by chemo-enzymatic Synergy
Supported by the National Natural Science Foundation of China (Grant No. 82430119), the research team led by Associate Professor Li Jian from Shanghai Jiao Tong University, in collaboration with the team of Professor Zhang Wei-Dong from the Naval Medical University/Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, has made progress in the study of total synthesis of terpenoid components in traditional Chinese medicine through chemoenzymatic synergistic catalysis. The study, entitled "Artificial farnesol epoxidase enables a concise synthesis of meroterpenoids," was published online in Science on August 15, 2025 (https://www.science.org/doi/10.1126/science.adt2096).
Terpenoids are a class of important active components in traditional Chinese medicine, possessing a wide range of biological activities and serving as a rich source for new drug discovery (e.g., artemisinin, paclitaxel, etc.). However, highly active components often have low natural abundance, severely impacting subsequent research and application. How to efficiently prepare low-abundance active components using alternative methods is a major challenge in current research.
To address this challenge, the team adopted a synergistic chem-bio synthesis strategy. They screened their self-built enzyme library to obtain the initial catalyst, EPO1, and through five rounds of saturation mutagenesis and directed evolution of EPO1, iteratively obtained EPO6—a highly precise "molecular scissor." EPO6 achieved 93% regioselectivity and 94% enantioselectivity for the internal olefin of farnesol, representing a substantial improvement over existing chemical catalysis methods (43% regioselectivity and 10% enantioselectivity). This achievement enables highly selective production of farnesol internal epoxide with excellent optical purity and decagram scale (a key biosynthetic precursor of terpenoids), allowing researchers to further leverage the higher reactivity of the remaining double bonds to construct various synthetic intermediates efficiently and concisely. On this basis, the team demonstrated a trimethylsilyl (TMS)-promoted mid-to-tail polyene tandem cyclization strategy, effectively addressing the energy barrier issues caused by bulky side chains during cyclization assembly. This strategy shortened the synthesis steps of 11 important natural drugs by 50%-74%, providing the most concise divergent synthetic route for the efficient preparation of immunosuppressants and antitumor terpenoid active components (Figure).
This research promotes a paradigm shift in the synthesis of scarce yet highly active natural products via synergistic chemoenzymatic catalysis. It moves the field beyond traditional dependence on—and minor modifications of—natural enzymes, toward an era in which synthetic chemists can rationally design and optimize tailored biocatalysts to meet specific strategic needs.
Contact Us

National Natural Science Foundation of China
Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@nsfc.gov.cn
