Progress in Research on human Monoclonal Antibodies Against Mpox Virus Achieved by Chinese Scholars
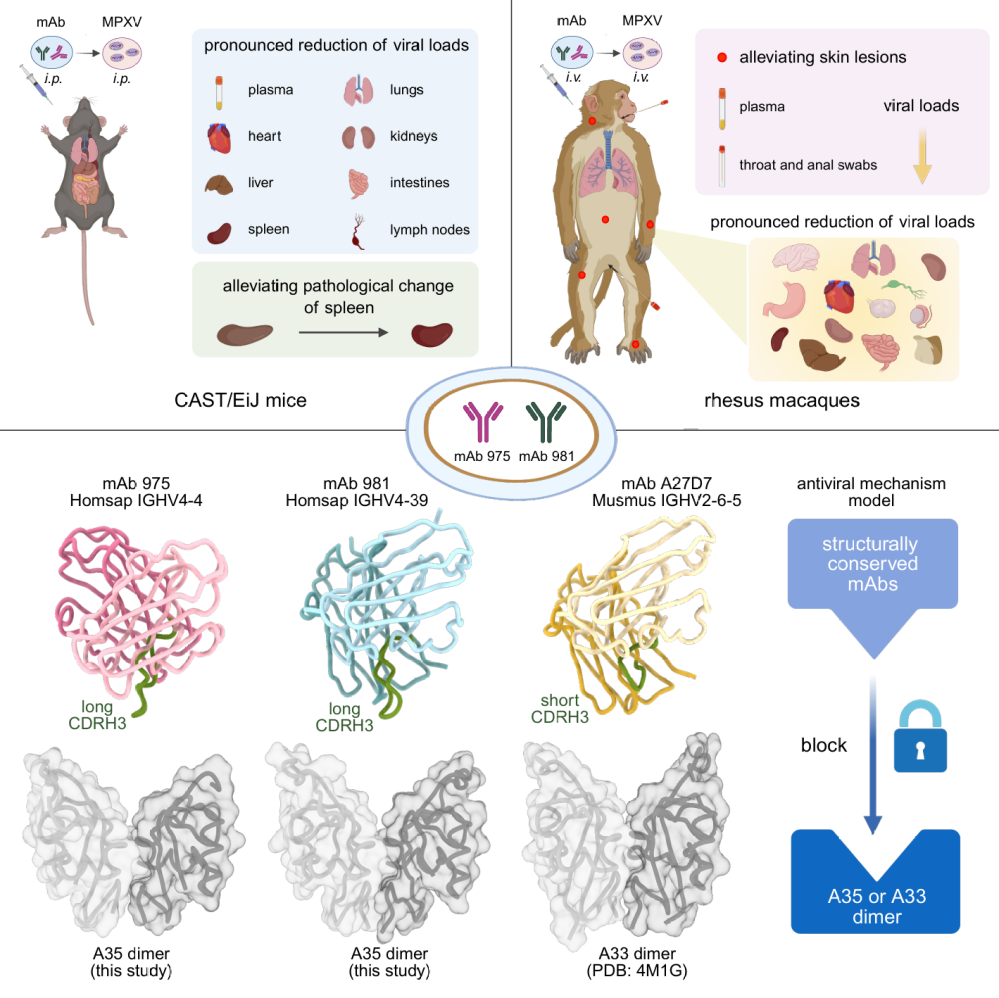
Figure: Structurally conserved human anti-A35 antibodies protect mice and macaques from mpox virus infection
Supported by the National Natural Science Foundation of China (Grant Nos.: 82025022, 82322040, 82222041, 82241068, 32422039) and other funding sources, the research team led by Professor Zheng Zhang and Bin Ju from the Second Affiliated Hospital of Southern University of Science and Technology/Shenzhen Third People's Hospital, Professor Jing Xue from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences, Professor Renhong Yan from the School of Medicine, Southern University of Science and Technology, and Professor Wenjie Tan from the Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention have collaborated to make progress in human monoclonal antibodies against the mpox virus. Their findings, titled "Structurally conserved human anti-A35 antibodies protect mice and macaques from mpox virus infection" were published online in the Cell on August 26, 2025. The paper is available at: https://www.sciencedirect.com/science/article/pii/S0092867425009195.
The mpox epidemic has posed a severe threat to global public health security, leading the World Health Organization (WHO) to declare it a Public Health Emergency of International Concern on two occasions. The A35 protein expressed on the enveloped virions (EVs) of the mpox virus is crucial for the intercellular transmission of the virus within the host, making it an ideal target for antiviral drugs and vaccines. Although previous studies have indicated the protective potential of antibodies targeting the A35 protein, data on their efficacy in non-human primate models, which more closely resemble humans, have been insufficient, and their molecular recognition mechanisms remained to be clarified.
The research team screened and validated two human monoclonal antibodies (mAbs), mAb 975 and mAb 981, which cross-react with both the A35 protein of the mpox virus and its homolog, the A33 protein of the vaccinia virus. In a CAST/EiJ mouse model of mpox virus infection, the administration of either antibody alone effectively reduced viral loads in plasma and multiple tissues and significantly mitigated pathological damage to the spleen. In a rhesus macaque model of mpox virus infection, these two antibodies also demonstrated significant protective effects, not only reducing viral levels in the macaques' plasma, pharyngeal swabs, anal swabs, and tissues but also markedly suppressing clinical symptoms such as skin lesions and pocks. Furthermore, the team employed cryogenic electron microscopy (cryo-EM) to resolve the high-resolution three-dimensional structures of the complexes formed by mAb 975 and mAb 981 with the A35 protein. Structural analysis revealed a cross-species conserved antibody binding mode: positively charged amino acids in the antibody's heavy-chain CDR3 region precisely insert into a groove-like structure in the middle of the antigen dimer, forming stable electrostatic interactions with negatively charged amino acids in that region. Concurrently, hydrophobic amino acids from multiple CDR loops of the antibody act synergistically with a hydrophobic platform on the antigen, further enhancing the stability of the antibody-antigen complex (Figure).
This study identifies two human monoclonal antibodies that effectively protect mice and rhesus macaques against mpox virus infection and elucidates the structural basis of the interaction between these antibodies and the mpox virus A35 protein. These findings provide promising candidates for the development of antibody-based drugs for mpox and offer a structural biology foundation and new strategies for the development of next-generation, broadly protective orthopoxvirus vaccines.
Contact Us

National Natural Science Foundation of China
Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@nsfc.gov.cn
