Progress Achieved by Chinese Scholars in the Development of Lithium metal batteries
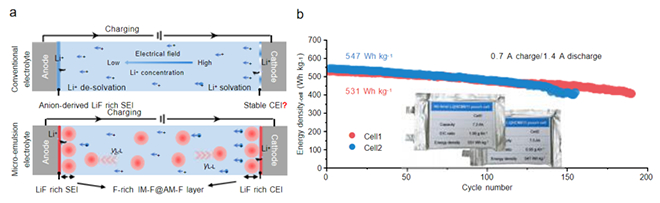
Fig. (a) Schematic of electrode/electrolyte interfacial evolution under different regulation strategies during charging; (b) Cycling performance of Li||NCM811 pouch cells with micro-emulsion electrolyte
Supported by the National Natural Science Foundation of China (Grant No. 92372207) and additional funding sources, the research team led by Prof. Jun Lu at Zhejiang University and collaborators has achieved a breakthrough in Lithium metal batteries. The team proposed a novel interfacial regulation mechanism based on liquid-liquid interfacial tension (γL–L), overcoming the industry challenge of simultaneously enhancing energy density and safety. The findings were published in Nature on July 16, 2025, under the title "Liquid-liquid interfacial tension stabilized Li metal batteries" Article link: https://doi.org/10.1038/s41586-025-09293-4
Next-generation battery systems beyond conventional technologies face critical challenges related to energy density, power density, safety, environmental adaptability, resource availability, and cost. As the most promising next-generation battery system, the industrialization of Lithium metal batteries is hindered by interfacial side reactions and safety risks. Traditional electrolyte designs fail to concurrently meet the stability requirements of high-voltage cathodes and compatibility with lithium metal anodes, making innovative interfacial regulation strategies essential.
To address this bottleneck, the team developed a new interfacial regulation strategy leveraging γL–L, inventing a novel microemulsion electrolyte. This innovation not only surpass the limitations of transcends traditional electrolyte component designs but also achieves dynamic synergistic stabilization of cathode/anode interfaces under high voltage through a physical field-driven mechanism. Molecular engineering enables perfluorinated solvents and partially fluorinated amphiphilic solvents to form microemulsion micelles (50-120 nm) via intermolecular interactions, uniformly dispersing the fluorinated phase in the electrolyte continuous phase. The γL–L driving force further guides functional components to migrate and enrich simultaneously at both electrode interfaces, constructing gradient-distributed fluorinated interfacial solvation layers (Fig.a). This field-direction/concentration-gradient-independent approach effectively decouples solvation structures from interfacial protection mechanisms, reduces contact between highly reactive solvents and electrodes, significantly suppresses interfacial side reactions, and enables dynamic co-protection of both interfaces.
Ah-level Li||NCM811 pouch cells employing this strategy demonstrate exceptional performance: energy density reaches 547/531 Wh kg-1, with capacity retention of 79%/81% after 155/189 cycles (Fig.b), representing industry-leading standards. Concurrently, gas generation during cycling is suppressed, and nail penetration tests show zero voltage drop and no fire, overcoming the safety limitations of high-energy-density batteries.
Contact Us

National Natural Science Foundation of China
Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@nsfc.gov.cn
