Chinese Scholars Have Made Progress in In-Situ Transmission Electron Microscopy Research on Lithium-Sulfur Batteries
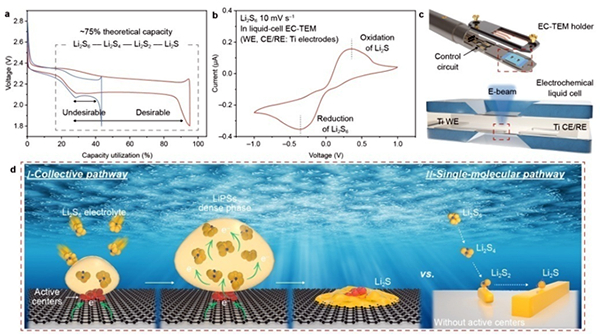
Figure (a) Comparison of charge/discharge profiles of desirable and undesirable Li2S6–Li2S reactions in Li-S batteries. (b) CV profile obtained in an electrochemical liquid cell at a scan rate of 10 mV s–1 showing the reduction of Li2S6 and oxidation of Li2S. (c) Configuration of liquid-cell EC-TEM. WE = working electrode, CE/RE = counter/reference electrodes. (d) Schematic illustration of electrochemical reactions of LiPSs at different electrode/electrolyte interfaces
With the support of the National Natural Science Foundation of China (grant numbers: 22288102, 2202100, U22A20396, 32101217, 21991151, 2191150, 91934303), Professors Hong-Gang Liao and Shi-Gang Sun from Xiamen University, along with the team of Professor Jian-Feng Chen from Beijing University of Chemical Technology, have established a high spatial-temporal resolution in situ liquid-phase electrochemical transmission electron microscopy (EC-TEM) through their autonomous development of homemade devices. This system has led to the discovery of a novel mechanism governing collective charge storage reactions in lithium-sulfur (Li-S) batteries. Their research findings, titled "Visualizing Interfacial Collective Reaction Behavior of Li-S Batteries," have recently been published in Nature (https://www.nature.com/articles/s41586-023-06326-8).
Under the impetus of the "carbon peak and carbon neutrality" objectives, the development of secondary battery systems with high energy density and high energy storage efficiency has become a current research hotspot. Unveiling the chemical reactions at the electrode and electrolyte interface at the atomic/molecular level is crucial for next-generation battery design. Presently, the understanding of potential mechanisms relies mainly on classical electrochemical theories and the Gouy-Chapman-Stern (GCS) double layer model at solid/liquid interfaces. In this model, it is generally assumed that reactants diffuse to the surface and undergo electrode reactions upon adsorption. However, the electrochemical reaction processes occurring at the electrode/electrolyte interface remain unclear, akin to a mysterious "black box." Li-S batteries, known for their extremely high energy density (2600 Wh kg–1) and low costs, continue to lack atomic/nanoscale insights into interface reactions due to challenges stemming from the limited spatial-temporal resolution of traditional in situ characterization tools and the instability and environmental sensitivity inherent to Li-S batteries.
In response to these challenges, the aforementioned research teams have established a high spatial-temporal resolution in situ liquid-phase EC-TEM technique. This groundbreaking approach couples a real ether-based electrolyte environment with externally applied electric fields to achieve dynamic real-time observation and investigation of atomic-scale interface reactions in Li-S batteries. The teams have discovered a unique interface reaction mechanism in Li-S batteries, involving the surface-induced gathering and collective charge storage of lithium polysulfides (LiPSs) through the introduction of metal nanocluster active centers. This phenomenon leads to the instantaneous transformation from LiPSs dense phases to non-equilibrium Li2S nanocrystallines. It differs from the classical pathway in traditional electrochemical reactions, where LiPSs gradually convert into Li2S2 and Li2S step by step. Molecular dynamics simulations have confirmed that long-range electrostatic interactions between active centers and LiPSs molecules lead to the formation of gathered molecular and collective electron transfer at the electrode interface. These results deepen the understanding of the electrochemical reaction interface mechanism in Li-S batteries and provide important theoretical references for the design of next-generation Li-S batteries.
Contact Us

National Natural Science Foundation of China
Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@nsfc.gov.cn
