Chinese Scientists and collaborators have made important progress on catalytic ring-cleavage of arene derivatives
With the support of National Natural Science Foundation of China (Nos. 21632001, 21772002, 81821004, 21933004), the research laboratory of Ning Jiao from the State Key Laboratory of Natural and Biomimetic Drugs of Peking University collaborated with K. N. Houk from the University of California, Los Angeles, and reported on the selective cleavage of arene rings via a biomimetic cascade activation strategy. The article entitled “Cleaving arene rings for acyclic alkenylnitrile synthesis” was published in Nature on July 19, 2021. Please find the paper at https://www.nature.com/articles/s41586-021-03801-y.
Synthetic chemistry is mainly built on the formation of carbon-carbon bonds. Conversely, selective C-C bond cleavage presents a formidable challenge, the solution of which will provide promising applications in synthesis, coal liquefaction, petroleum cracking, polymer degradation, and biomass conversion. The aromatic rings are ubiquitous in inert chemical feed stocks. The preparation of useful C6 synthons and more complex acyclic fragments from aromatic rings is attractive; however, due to their aromaticity and high bond-dissociation energy, catalytic methods of arene cleavage under mild conditions with good selectivity had hitherto remained unexplored.
Inspired by enzymatic processes in bacteria, Jiao et al. demonstrated a novel strategy of copper-catalyzed aerobic oxidative aromatic ring-opening that affords selective C-C bond cleavage, which was further applied in the modification of polycyclic aromatics and complex molecules (Fig. 1). This unprecedented method can be employed to efficiently convert a broad range of arene derivatives into alkenyl nitriles, industrially important adiponitrile, hexamethylenediamine, adipic acid derivatives, etc. With the experimental studies and quantum mechanical calculations using density functional theory (DFT, conducted by the Houk group ), the authors revealed a mechanism of cascade activation of inert aromatic C-C bond. As a part of continuous endeavors in oxidative C-C bond cleavage in the Jiao research laboratory, this discovery would henceforth pave the way for effective transformation of erstwhile inactive arenes.
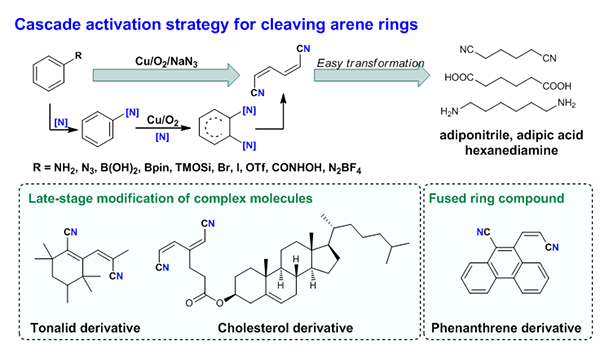
Fig. 1 Cascade activation strategy for arene cleavage and its applications.
Dr. Shaodong Zhang, Dr. Rong Zhou, and Dr. Xuefeng Fu
Contact Us

National Natural Science Foundation of China
Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@nsfc.gov.cn
